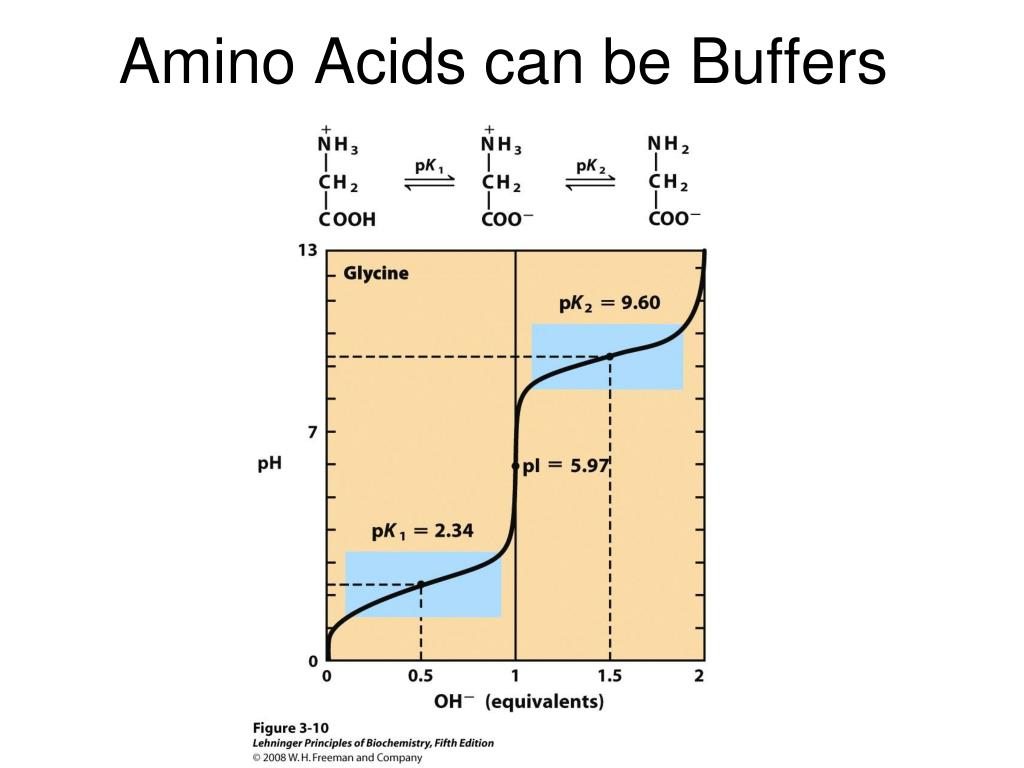
Amino Acids Chemical Buffer . Free amino acids in cells also contribute to buffering. The zwitterion of an amino acid exists at a. At the “center” of each amino acid is a carbon. all amino acids have the same basic structure, which is shown in figure 2.1. when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. In aqueous solution, an h + ion is therefore transferred from. an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. Draw fisher projections and assign d/l or r/s. all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon called the α carbon and attached to it.
from www.slideserve.com
At the “center” of each amino acid is a carbon called the α carbon and attached to it. all amino acids have the same basic structure, which is shown in figure 2.1. In aqueous solution, an h + ion is therefore transferred from. Draw fisher projections and assign d/l or r/s. Free amino acids in cells also contribute to buffering. At the “center” of each amino acid is a carbon. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. The zwitterion of an amino acid exists at a. all amino acids have the same basic structure, which is shown in figure 2.1. when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge.
PPT Amino Acids and Peptides PowerPoint Presentation, free download ID6333061
Amino Acids Chemical Buffer Free amino acids in cells also contribute to buffering. when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. At the “center” of each amino acid is a carbon. At the “center” of each amino acid is a carbon called the α carbon and attached to it. Free amino acids in cells also contribute to buffering. In aqueous solution, an h + ion is therefore transferred from. Draw fisher projections and assign d/l or r/s. all amino acids have the same basic structure, which is shown in figure 2.1. all amino acids have the same basic structure, which is shown in figure 2.1. The zwitterion of an amino acid exists at a. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing.
From www.numerade.com
SOLVED Q The buffer systems that are the most important in the Intracellular fluid are Amino Acids Chemical Buffer an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. Free amino acids in cells also contribute to buffering. The zwitterion of an amino acid exists at a. At the “center” of each amino acid is a carbon. Draw fisher projections and assign d/l or r/s.. Amino Acids Chemical Buffer.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Walkthrough|AQA A Level Chemistry Amino Acids Chemical Buffer all amino acids have the same basic structure, which is shown in figure 2.1. Draw fisher projections and assign d/l or r/s. At the “center” of each amino acid is a carbon called the α carbon and attached to it. In aqueous solution, an h + ion is therefore transferred from. At the “center” of each amino acid is. Amino Acids Chemical Buffer.
From www.slideserve.com
PPT Amino Acids and Peptides PowerPoint Presentation, free download ID6333061 Amino Acids Chemical Buffer all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon. The zwitterion of an amino acid exists at a. all amino acids have the same basic structure, which is shown in figure 2.1. Draw fisher projections and assign d/l or r/s. At the “center”. Amino Acids Chemical Buffer.
From www.youtube.com
Types of buffers carbonic acid buffer phosphate buffer protein amino acids buffers botany D Amino Acids Chemical Buffer Free amino acids in cells also contribute to buffering. when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. all amino acids have the same basic structure, which is shown in figure 2.1. all amino acids have the same basic structure,. Amino Acids Chemical Buffer.
From www.youtube.com
Protein Buffering System YouTube Amino Acids Chemical Buffer Free amino acids in cells also contribute to buffering. The zwitterion of an amino acid exists at a. all amino acids have the same basic structure, which is shown in figure 2.1. Draw fisher projections and assign d/l or r/s. when an amino acid contains both a plus and a minus charge in the backbone, it is called. Amino Acids Chemical Buffer.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Amino Acids Chemical Buffer an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. Draw fisher projections and assign d/l or r/s. Free amino acids in cells also contribute to buffering. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic. Amino Acids Chemical Buffer.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 Amino Acids Chemical Buffer Draw fisher projections and assign d/l or r/s. At the “center” of each amino acid is a carbon called the α carbon and attached to it. The zwitterion of an amino acid exists at a. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. all. Amino Acids Chemical Buffer.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry Amino Acids Chemical Buffer At the “center” of each amino acid is a carbon. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. In aqueous solution, an h + ion is therefore transferred from. Draw fisher projections and assign d/l or r/s. The zwitterion of an amino acid exists at. Amino Acids Chemical Buffer.
From studylib.net
acid base buffers Amino Acids Chemical Buffer an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. At the “center” of each amino acid is a carbon. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. In aqueous solution,. Amino Acids Chemical Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Amino Acids Chemical Buffer all amino acids have the same basic structure, which is shown in figure 2.1. In aqueous solution, an h + ion is therefore transferred from. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. all amino acids have the same basic structure, which is. Amino Acids Chemical Buffer.
From labchem-wako.fujifilm.com
Buffer for HighSpeed Amino Acid Analyzer PFSET・02019571[Detail Information] [Analytical Amino Acids Chemical Buffer Draw fisher projections and assign d/l or r/s. an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. Free amino acids in cells. Amino Acids Chemical Buffer.
From www.studypool.com
SOLUTION Amino acids as acids bases and buffers Studypool Amino Acids Chemical Buffer all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon called the α carbon and attached to it. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. Free amino acids in. Amino Acids Chemical Buffer.
From chem.libretexts.org
1.5 Water, Equilibrium, and Buffers Chemistry LibreTexts Amino Acids Chemical Buffer At the “center” of each amino acid is a carbon called the α carbon and attached to it. Free amino acids in cells also contribute to buffering. In aqueous solution, an h + ion is therefore transferred from. an amino acid can act as a buffer because it can react with added acids and bases to keep the ph. Amino Acids Chemical Buffer.
From www.youtube.com
Properties of buffers Acids and bases AP Chemistry Khan Academy YouTube Amino Acids Chemical Buffer an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. At the “center” of each amino acid is a carbon. all amino acids have the same basic structure, which is shown in figure 2.1. Draw fisher projections and assign d/l or r/s. The zwitterion of. Amino Acids Chemical Buffer.
From mungfali.com
Amino Acid Titration Curve Amino Acids Chemical Buffer all amino acids have the same basic structure, which is shown in figure 2.1. all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon. In aqueous solution, an h + ion is therefore transferred from. when an amino acid contains both a plus. Amino Acids Chemical Buffer.
From labchem-wako.fujifilm.com
Buffer for HighSpeed Amino Acid Analyzer PHSET・02819511[Detail Information] [Analytical Amino Acids Chemical Buffer when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Draw fisher projections and assign d/l or r/s. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms, existing. an. Amino Acids Chemical Buffer.
From www.studypool.com
SOLUTION Amino Acids as Acids, Bases and Buffers Detail lecture & Notes Studypool Amino Acids Chemical Buffer Free amino acids in cells also contribute to buffering. At the “center” of each amino acid is a carbon called the α carbon and attached to it. an amino acid can act as a buffer because it can react with added acids and bases to keep the ph nearly constant. Draw fisher projections and assign d/l or r/s. . Amino Acids Chemical Buffer.
From www.savemyexams.com
Amino Acids AQA A Level Chemistry Revision Notes 2017 Amino Acids Chemical Buffer Draw fisher projections and assign d/l or r/s. The zwitterion of an amino acid exists at a. when an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. At the “center” of each amino acid is a carbon. an amino acid can act. Amino Acids Chemical Buffer.